



Blog
Hot Category
Latest Blog
06 Jan 2025
Eli
Lithium Iron Phosphate (LiFePO4 or LFP) batteries have become a cornerstone in energy storage and electric mobility due to their safety, longevity, and eco-friendliness. But what makes these batteries unique? Let’s dive into the chemistry behind LiFePO4 batteries to understand their structure, function, and advantages.
LiFePO4 is a type of lithium-ion battery, where the cathode material is lithium iron phosphate. The chemical formula of this material is LiFePO4, and it is classified as a lithium iron phosphate compound belonging to the olivine group.
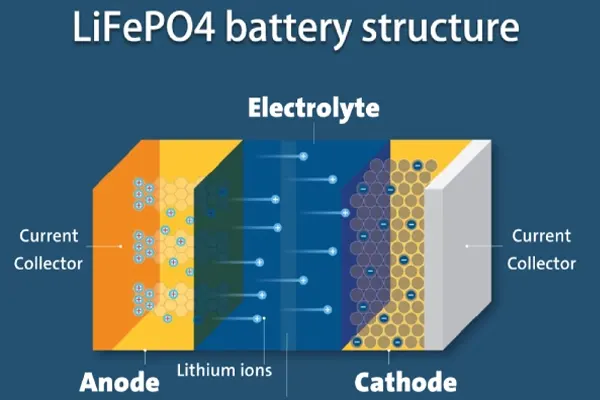
The primary electrochemical reactions in LiFePO4 batteries involve the reversible intercalation and deintercalation of lithium ions (Li+) between the cathode and the anode. Here is a breakdown of the key components and their roles:
Cathode (LiFePO4): This serves as the source of lithium ions. During discharge, lithium ions move from the cathode to the anode through the electrolyte, and during charging, the reverse happens.
Anode (Typically Graphite): The anode stores lithium ions when the battery is charged and releases them during discharge.
Electrolyte: This medium facilitates the movement of lithium ions between the anode and cathode. It is typically a lithium salt dissolved in an organic solvent.
Separator: This physically separates the anode and cathode to prevent short circuits while allowing lithium ions to pass through.
The fundamental reactions in a LiFePO4 battery can be represented as follows:
Lithium ions and electrons leave the LiFePO4 cathode. The lithium ions travel through the electrolyte to the anode, while the electrons flow through an external circuit, providing power.
Lithium ions return to the cathode, recombining with electrons, and restoring the material to its original LiFePO4 state.
Safety: LiFePO4 is thermally stable and less prone to thermal runaway compared to other lithium-ion chemistries like LiCoO2. This makes it safer for use in large-scale applications.
Long Cycle Life: The strong P-O bonds in the phosphate structure contribute to the excellent stability of LiFePO4, allowing thousands of charge and discharge cycles with minimal capacity loss.
Eco-Friendliness: The absence of toxic and rare materials such as cobalt makes LiFePO4 batteries more environmentally friendly.
High Discharge Rates: LiFePO4 batteries can deliver high currents without significant degradation, making them ideal for applications requiring high power.
Thanks to their unique chemical properties, LiFePO4 batteries are widely used in: Electric vehicles Energy storage equipment Portable power tools Backup power systems
The chemistry of LiFePO4 batteries strikes a balance between safety, performance, and environmental impact. By understanding the underlying principles, we can appreciate why these batteries are becoming a preferred choice for sustainable energy solutions. As research continues, the performance of LiFePO4 batteries is likely to improve further, cementing their role in the future of energy storage.